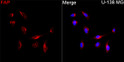
| Host: | Rabbit |
| Applications: | IF/ICC/ELISA |
| Reactivity: | Human |
| Note: | STRICTLY FOR FURTHER SCIENTIFIC RESEARCH USE ONLY (RUO). MUST NOT TO BE USED IN DIAGNOSTIC OR THERAPEUTIC APPLICATIONS. |
| Clonality : | Polyclonal |
| Conjugation: | Unconjugated |
| Isotype: | IgG |
| Formulation: | PBS with 0.05% Proclin300, 50% Glycerol, pH 7.3. |
| Purification: | Affinity purification |
| Concentration: | Lot specific |
| Dilution Range: | IF/ICC:1:50-1:200 ELISA:Recommended starting concentration is 1 Mu g/mL. Please optimize the concentration based on your specific assay requirements. |
| Gene Symbol: | FAP |
| Gene ID: | 2191 |
| Uniprot ID: | SEPR_HUMAN |
| Immunogen Region: | 481-580 aa |
| Specificity: | A synthetic peptide corresponding to a sequence within amino acids 481-580 of human FAP (NP_004451.2). |
| Immunogen Sequence: | TDQEIKILEENKELENALKN IQLPKEEIKKLEVDEITLWY KMILPPQFDRSKKYPLLIQV YGGPCSQSVRSVFAVNWISY LASKEGMVIALVDGRGTAFQ |
| Tissue Specificity | Expressed in adipose tissue. Expressed in the dermal fibroblasts in the fetal skin. Expressed in the granulation tissue of healing wounds and on reactive stromal fibroblast in epithelial cancers. Expressed in activated fibroblast-like synoviocytes from inflamed synovial tissues. Expressed in activated hepatic stellate cells (HSC) and myofibroblasts from cirrhotic liver, but not detected in normal liver. Expressed in glioma cells (at protein level). Expressed in glioblastomas and glioma cells. Isoform 1 and isoform 2 are expressed in melanoma, carcinoma and fibroblast cell lines. |
| Post Translational Modifications | N-glycosylated. The N-terminus may be blocked. |
| Function | Cell surface glycoprotein serine protease that participates in extracellular matrix degradation and involved in many cellular processes including tissue remodeling, fibrosis, wound healing, inflammation and tumor growth. Both plasma membrane and soluble forms exhibit post-proline cleaving endopeptidase activity, with a marked preference for Ala/Ser-Gly-Pro-Ser/Asn/Ala consensus sequences, on substrate such as alpha-2-antiplasmin SERPINF2 and SPRY2. Degrade also gelatin, heat-denatured type I collagen, but not native collagen type I and IV, vitronectin, tenascin, laminin, fibronectin, fibrin or casein. Also has dipeptidyl peptidase activity, exhibiting the ability to hydrolyze the prolyl bond two residues from the N-terminus of synthetic dipeptide substrates provided that the penultimate residue is proline, with a preference for Ala-Pro, Ile-Pro, Gly-Pro, Arg-Pro and Pro-Pro. Natural neuropeptide hormones for dipeptidyl peptidase are the neuropeptide Y (NPY), peptide YY (PYY), substance P (TAC1) and brain natriuretic peptide 32 (NPPB). The plasma membrane form, in association with either DPP4, PLAUR or integrins, is involved in the pericellular proteolysis of the extracellular matrix (ECM), and hence promotes cell adhesion, migration and invasion through the ECM. Plays a role in tissue remodeling during development and wound healing. Participates in the cell invasiveness towards the ECM in malignant melanoma cancers. Enhances tumor growth progression by increasing angiogenesis, collagen fiber degradation and apoptosis and by reducing antitumor response of the immune system. Promotes glioma cell invasion through the brain parenchyma by degrading the proteoglycan brevican. Acts as a tumor suppressor in melanocytic cells through regulation of cell proliferation and survival in a serine protease activity-independent manner. |
| Protein Name | Prolyl Endopeptidase Fap 170 Kda Melanoma Membrane-Bound Gelatinase Dipeptidyl Peptidase Fap Fibroblast Activation Protein Alpha Fapalpha Gelatine Degradation Protease Fap Integral Membrane Serine Protease Post-Proline Cleaving Enzyme Serine Integral Membrane Protease Simp Surface-Expressed Protease Seprase Cleaved Into - Antiplasmin-Cleaving Enzyme Fap - Soluble Form Apce |
| Cellular Localisation | Prolyl Endopeptidase Fap: Cell Surface Cell Membrane Single-Pass Type Ii Membrane Protein Cell Projection Lamellipodium Membrane Invadopodium Membrane Ruffle Membrane Membrane Localized On Cell Surface With Lamellipodia And Invadopodia Membranes And On Shed Vesicles Colocalized With Dpp4 At Invadopodia And Lamellipodia Membranes Of Migratory Activated Endothelial Cells In Collagenous Matrix Colocalized With Dpp4 On Endothelial Cells Of Capillary-Like Microvessels But Not Large Vessels Within Invasive Breast Ductal Carcinoma Anchored And Enriched Preferentially By Integrin Alpha-3/Beta-1 At Invadopodia Plasma Membrane Protrusions That Correspond To Sites Of Cell Invasion In A Collagen-Dependent Manner Localized At Plasma And Ruffle Membranes In A Collagen-Independent Manner Colocalized With Plaur Preferentially At The Cell Surface Of Invadopodia Membranes In A Cytoskeleton- Integrin- And Vitronectin-Dependent Manner Concentrated At Invadopodia Membranes Specialized Protrusions Of The Ventral Plasma Membrane In A Fibrobectin-Dependent Manner Colocalizes With Extracellular Components (Ecm) Such As Collagen Fibers And Fibronectin Antiplasmin-Cleaving Enzyme Fap Soluble Form: Secreted Found In Blood Plasma And Serum Isoform 2: Cytoplasm |
| Alternative Antibody Names | Anti-Prolyl Endopeptidase Fap antibody Anti-170 Kda Melanoma Membrane-Bound Gelatinase antibody Anti-Dipeptidyl Peptidase Fap antibody Anti-Fibroblast Activation Protein Alpha antibody Anti-Fapalpha antibody Anti-Gelatine Degradation Protease Fap antibody Anti-Integral Membrane Serine Protease antibody Anti-Post-Proline Cleaving Enzyme antibody Anti-Serine Integral Membrane Protease antibody Anti-Simp antibody Anti-Surface-Expressed Protease antibody Anti-Seprase Cleaved Into - Antiplasmin-Cleaving Enzyme Fap - Soluble Form antibody Anti-Apce antibody Anti-FAP antibody |
Information sourced from Uniprot.org